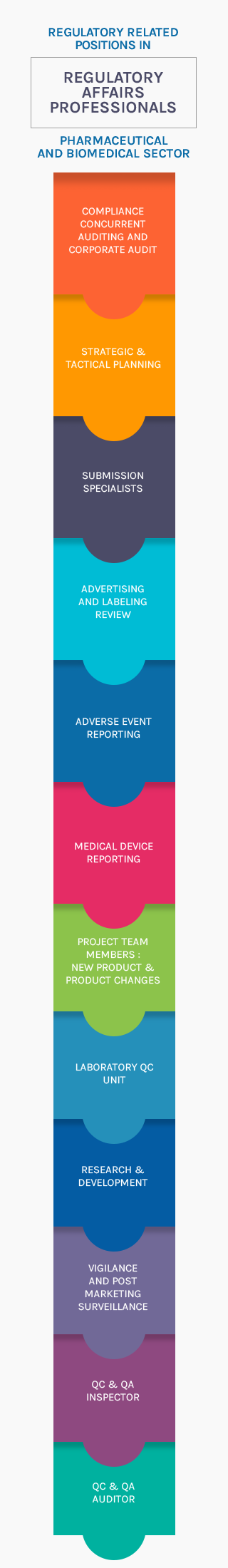
Regulatory Affairs
"Procyon" has well experienced and committed team of regulatory personnel.
- Qualified and Trained Personnel
- Filing of Drug Master Files
- Filing of Certificate of Suitability for EP listed products
- Response to Regulatory and Customer Queries
- Ensure manufacturing as per Regulatory requirements
- Conducting audits prior to submissions of Master Files